In cell culture work, precise numbers govern outcomes—from growth rate to media stability. This guide highlights Useful Numbers For Cell Culture that every lab should track to improve reproducibility and data quality. By understanding these constants, you can design better experiments, compare results across runs, and communicate methods with clarity.
Core constants you should know
These foundational figures shape everyday decisions in the lab, from setting up incubators to choosing passage schedules. Treat them as a living reference you revisit when planning experiments or evaluating data.
Key Points
- Maintain incubator conditions around 37°C with 5% CO2 and high humidity to stabilize pH, a cornerstone of Useful Numbers For Cell Culture.
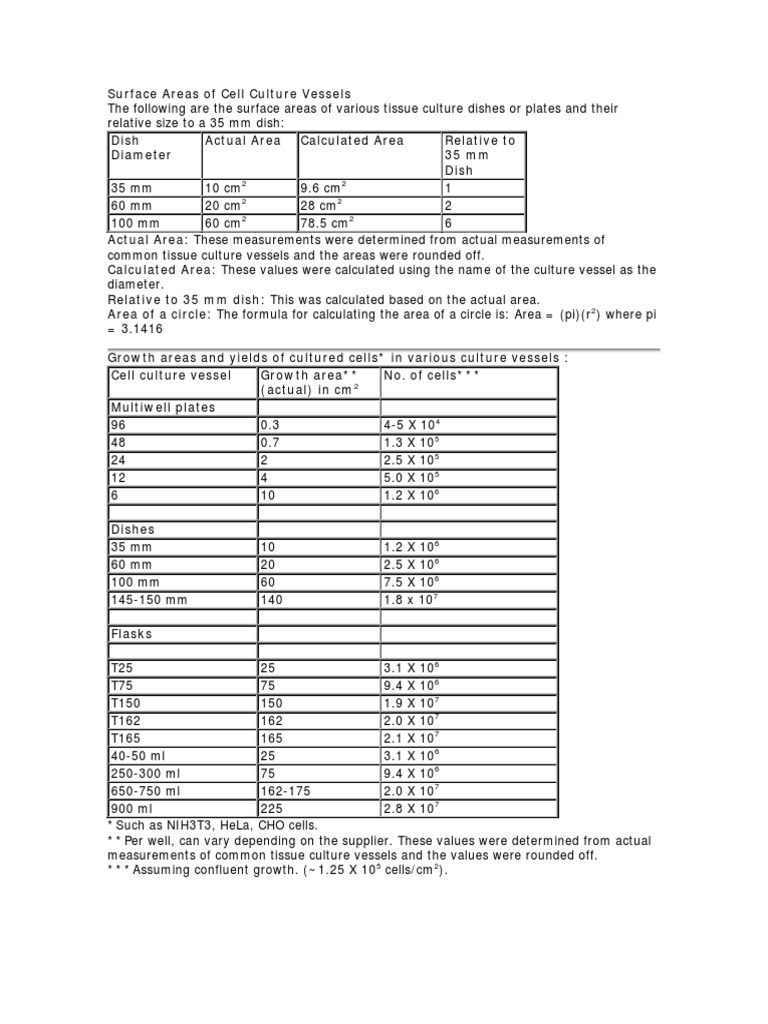
- Track cell density and passage number to minimize variability between culture batches.
- Document media composition with precise concentrations rather than approximate values to reduce lot-to-lot differences.
- Record timing metrics for feeding and passaging to keep growth curves consistent across experiments.
- Validate key numeric parameters periodically with quick checks (pH, osmolality, and viability) to catch drift early.
How temperature, CO2, and humidity influence useful numbers for cell culture
The values above are not merely numbers; they represent a delicate balance that sustains cell health. Temperature drives metabolic rate, CO2 maintains the bicarbonate-buffered pH, and humidity minimizes evaporation. When these constants drift, the resulting changes ripple through growth curves, drug responses, and differentiation signals. Keeping temperature near 37°C, CO2 near 5%, and humidity high enough to prevent evaporation helps ensure that data across runs remain comparable.
Recording and reporting constants for better reproducibility
Adopt a consistent approach to capture and share numbers. Use units that are unambiguous (e.g., °C for temperature, % for CO2, cells/mL for density) and include instrument calibration details. In publications or lab notebooks, pair each constant with the context it affects (cell line, medium, and passage) so others can interpret results accurately.
What are the most important numbers to track daily in a cell culture lab?
+Daily tracking typically includes incubator temperature (°C), CO2 level (%), humidity, cell density (cells/mL or cells/cm²), passage number, medium change timing, and any observed viability. Keeping these aligned with your standard ensures consistency across experiments.
How should I report numeric constants in lab notebooks or papers?
+Report constants with standardized units, include calibration details for instruments, and note the specific cell line and media used. Consider adding a concise Methods box or supplementary table that lists all key numbers alongside their context.
What happens if these numbers drift over time?
+Drift can alter growth rates, differentiation, and treatment responses, leading to inconsistent results between experiments or labs. Regular calibration and quick checks help detect drift early, allowing timely corrective action.
How often should I validate core constants?
+Validation frequency depends on usage, but a practical approach is to verify key constants weekly, with additional checks whenever there is a reagent change, instrument recalibration, or noticeable shifts in culture behavior.