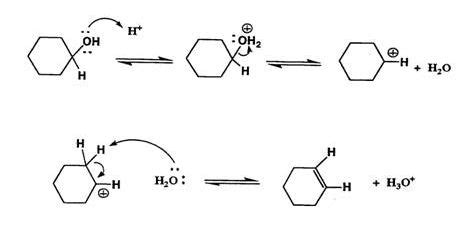
Solovolysis Of 3Acetate is a fundamental step in many organic transformations where an acetate ester undergoes solvolytic cleavage or rearrangement under designed solvent conditions. In practice, researchers frequently encounter a set of common problems that can limit conversion, selectivity, or product stability. This article explores those typical challenges and provides practical, high-level guidance on how to address them, focusing on clarity and applicability for day-to-day interpretation of Solovolysis Of 3Acetate.
Key Points
- Solovolysis Of 3Acetate performance hinges on substrate accessibility and the chosen solvent system's ability to stabilize transition states.
- Solvent polarity and hydrogen-bonding capacity can dramatically influence reaction rate and selectivity in Solovolysis Of 3Acetate.
- Purity of starting material and absence of competing ester groups are crucial to minimize side pathways.
- Analytical monitoring, even at a high level, helps distinguish complete conversion from partial progress in Solovolysis Of 3Acetate.
- Careful consideration of conditions that balance reactivity with product stability is key to avoiding decomposition during Solovolysis Of 3Acetate.
Common Problems In Solovolysis Of 3Acetate
Problem 1: Incomplete Solvolysis
In many cases, Solovolysis Of 3Acetate does not reach full conversion under the chosen conditions. Potential causes include limited solvent penetration, steric hindrance near the acetate group, or competing stabilizations of intermediates. The result is a mixture with unreacted starting material and partially converted products.
Fix: Reassess the reaction environment from a high-level perspective. Consider whether a solvent system with better polarity or specific solvation characteristics could facilitate the process, and evaluate substrate purity and reaction duration without pushing the system toward decomposition. Employ non-destructive monitoring to gauge progress over time.
Problem 2: Unwanted Side Reactions
Unintended pathways such as hydrolysis of other ester functionalities, transesterification, or rearrangements can compete with Solovolysis Of 3Acetate, especially in molecules with multiple labile sites. These side reactions reduce yield and complicate product isolation.
Fix: Prioritize selectivity by considering protective strategies for sensitive groups and choosing solvent components that discourage competing pathways. Minimize impurities and avoid additives that can promote alternate ester exchanges or rearrangements.
Problem 3: Decomposition Under Solvolysis Conditions
Some substrates or intermediates in Solovolysis Of 3Acetate are sensitive to the operational environment and can degrade under the energetic or acidic/base-rich conditions often used in solvolysis. Decomposition manifests as loss of material, lower mass balance, or formation of complex byproducts.
Fix: Favor milder reaction environments where possible and balance the energy input with the substrate’s stability profile. Quick quenching after a qualitative sense of completion and avoiding prolonged exposure to reactive conditions can help preserve product integrity.
Problem 4: Solvent Incompatibility or Phase Issues
Immiscible or poorly miscible solvent systems hinder efficient mass transfer, limiting contact between reactants and the solvolysis medium. Phase separation can also trap reactants in one phase, reducing overall conversion.
Fix: Reevaluate the solvent system for better miscibility and substrate solubility, potentially using a co-solvent approach that maintains compatibility with the solvolysis mechanism. Manage phase behavior to enhance interfacial contact without promoting unwanted side reactions.
Problem 5: Impurity Sensitivity
Trace impurities—acids, bases, or contaminants—can alter reaction pathways, catalyze side reactions, or destabilize reactive intermediates in Solovolysis Of 3Acetate. Even small amounts of impurities can have outsized effects on outcome.
Fix: Emphasize purification of starting materials and maintain clean reaction conditions. Pre-treat materials to remove inhibitors or contaminants that can distort the reaction landscape, and implement baseline checks for trace impurities before initiating solvolysis.
Practical guidelines for optimizing Solovolysis Of 3Acetate
To minimize the issues discussed above, focus on conceptual optimization: ensure overall substrate accessibility, align solvent properties with the reaction’s polar and stabilizing needs, and keep impurities to a minimum. Regular, non-destructive monitoring helps you form a practical sense of progress and prevents overexposure of sensitive products. In practice, balancing reactivity with product stability is often the most reliable route to reliable outcomes in Solovolysis Of 3Acetate.
What are the most common reasons Solovolysis Of 3Acetate fails to proceed?
+Common reasons include limited substrate accessibility, suboptimal solvent polarity, and the presence of impurities that disturb the reaction environment. A broad, non-operational review of the solvent system and material quality often reveals the primary cause.
<div class="faq-item">
<div class="faq-question">
<h3>How does solvent choice influence Solovolysis Of 3Acetate?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>Solvent properties such as polarity, dielectric constant, and hydrogen-bonding ability can affect reaction rate and selectivity. Selecting a solvent that stabilizes key intermediates without promoting side reactions is a high-level consideration to improve outcomes.</p>
</div>
</div>
<div class="faq-item">
<div class="faq-question">
<h3>Can impurities affect Solovolysis Of 3Acetate, and how can I minimize their impact?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>Yes, trace impurities can steer the reaction toward undesired pathways. Minimizing their impact involves purifying starting materials, avoiding sensitive additives, and maintaining clean reaction conditions to preserve the intended solvolysis pathway.</p>
</div>
</div>
<div class="faq-item">
<div class="faq-question">
<h3>What non-destructive indicators help assess progress in Solovolysis Of 3Acetate?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>Non-destructive indicators include qualitative spectral shifts, changes in polarity-sensitive signals, or monitoring of overall material balance through gentle analytical methods. These approaches help decide whether to continue or halt solvolysis without consuming the sample.</p>
</div>
</div>